Abstract
Background Chronic red blood cell transfusion therapy (CTT) is an integral component of the management of Transfusion Dependent Thalassemia (TDT) and Sickle Cell Disease (SCD). CTT requires time and significantly impacts the quality of life of patients with TDT and SCD. In some cases, the environment in which transfusions are performed is uncomfortable and doesn't offer adequate spaces and facilities. And as a result, the patient experiences a condition of discomfort and tries with his own means to get distracted or "estranged". Hence, attention should be paid to psychological, emotional and social needs of patients on CTT in order to reduce the burden of the procedure with activities that are capable of engaging more the person.
Aims From this need and the opportunities offered by the digital technologies available today, the AREAL project was designed by the Italian startup Softcare Studios in partnership with the pharma company Novartis and in collaboration with hemoglobinopathy specialized medical staff. Objectives of this pilot project were: i) collect feedback from patients with TDT and SCD and from healthcare professional workers regarding utility and feasibility of AREAL; ii) detect and evaluate changing in states of patient's anxiety and depression.
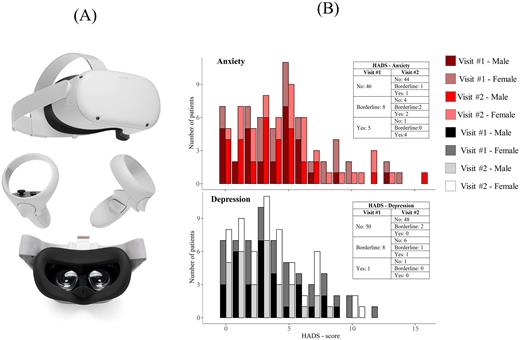
Methods AREAL consists of three gaming activities enjoyed in virtual reality thanks to the use of suitable Virtual Reality (VR) viewers (Figure 1A) developed to immerse the patient in a sensorial stimulating scenario and designed to stimulate cognitive skills: activity 1 for short-term memory, activity 2 for task switching, activity 3 for problem solving. AREAL also integrates elements of patient education in therapy and recommended lifestyle habits to promote their well-being. Anxiety and depression were assessed by a self-assessment questionnaire, the Hospital Anxiety and Depression Scale (HADS), administered after each virtual session.
Results The pilot group was composed by 70 subjects on CTT followed in 3 hospital centers in Italy (Genova, Napoli, Padova). The 57% were males, median age was 31 years (IQR: 21-40), 53 patients were affected by TDT and 6 by SCD. The total number of sessions collected is 129; 59 patients experienced two sessions and were included in the analysis for HADS. The average duration of game sessions lasted 17 minutes.Activity 1 was the most played and most rarely interrupted, followed by 2 and finally by 3, where 50% of sessions are been interrupted due to its difficulty. In Activity 1 patients recognized the goal of the game, which is to stimulate memory, and they liked its immediacy. Excessive facility, especially considering the repetition over time during the therapy, was emphasized by patients who suggested making the game more complicated. Compared to Activity 1, Activity 2 is more complicated and engaging, emerging as the overall favorite of the study patients. Undoubtedly, Activity 3 emerged as the most difficult one, both to play and to understand, also given its focus on problem solving. Based on the feedback from healthcare professionals learning to use the virtual application wasn't particularly difficult. The experience of the staff confirmed some of the critical issues also communicated by patients (such as the high difficulty of the third game activity and request for greater variability over time).Anxiety and depression were detected by HADS respectively in 13 and in 9 pts after first session, in 10 and in 4 pts after second session, suggesting a possible benefit of VR; the number of patients with borderline profile reduced from 8 to 3 both for anxiety and depression (Figure 1B). There were no significant differences of scores and/or percentages between sessions.
Conclusions There is a significant need to iterate AREAL on the basis of different age groups, each with specific needs, preferences and level of confidence with technologies. From the collected feedback, AREAL was perceived as an innovative project and an opportunity to pay attention to the critical issues of the patients' therapeutic experience on CTT. However, it is needed to optimize the experience in order to free the operator from extra commitments as much as possible that could impact the quality of hospital work routine. AREAL seems to be an opportunity to reduce discomfort and anxiety during CCT in the subset of pts with a profile borderline for anxiety and depression but needs to tailor the intervention to specific subsets of patients.
Disclosures
Megale:Novartis: Consultancy; Softcare studios: Current Employment. Casale:Novartis: Honoraria, Speakers Bureau; Biovalley: Honoraria, Speakers Bureau; Agios: Membership on an entity's Board of Directors or advisory committees. Colombatti:Novartis: Membership on an entity's Board of Directors or advisory committees; Novonordisk: Membership on an entity's Board of Directors or advisory committees; Addmedica: Membership on an entity's Board of Directors or advisory committees; Forma Therapeutics: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees; Vertex: Membership on an entity's Board of Directors or advisory committees; Bluebirdbio: Research Funding; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees. Pinto:Novartis: Honoraria, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees; Bluebirdbio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Vertex: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Biovalley: Honoraria, Speakers Bureau. Forni:BMS (Celgene): Consultancy, Research Funding, Speakers Bureau; Vertex: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau. Perrotta:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal